Adventist doctor among first to use breakthrough technology for cardiac arrest
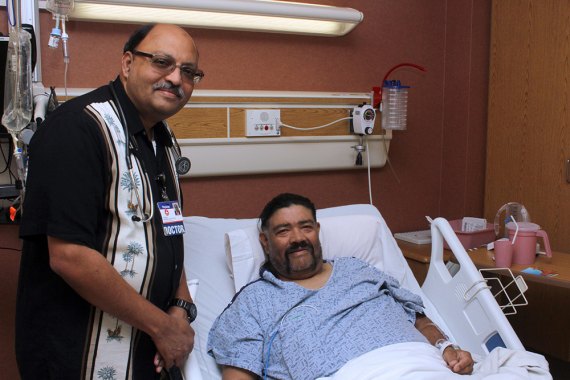
On March 19, Dr. Gavini implanted cardioverter defibrillators just under the skin of two patients. According to Boston Scientific, the S-ICD (subcutaneous implantable cardioverter defibrillator) System is the world’s first and only implantable defibrillator that provides protection from sudden cardiac arrest without touching the heart. It’s different from a transvenous TV-ICD System, which is implanted in the chest and has lead wires inside the heart, offering pacemaker functions for patients who need it.
The new S-ICD System is implanted under the skin outside the rib cage, with an electrode placed next to the breast bone or sternum. It constantly monitors the heart so that when a lethal rhythm occurs, the electrode delivers a shock to the heart, similar to external paddles used by paramedics. The shock can restore the heart’s normal rhythm.
“It’s like having an ambulance in your body, monitoring your heart and giving you a shock when you need it,” says Dr. Gavini. “This is a life-saving procedure that is safe and can add years to a person’s life.”
Both patients who had the procedure performed receive care from Community Care – Hanford.
One of the patients, Mariano Montoya, 54 of Corcoran, recently had open heart surgery and triple bypass surgery on May 20, 2014. He was thrilled he was a candidate for the S-ICD procedure.
“It feels good to know I will live a longer life,” says Montoya, who used to be a security officer. “I can’t do anymore heavy-lifting, but it’s nice to know I have this life-saving technology on my side, instead of in my heart.”
Kenneth Day of Hanford was the second patient to have the procedure performed on March 19. He has coronary artery disease and has suffered a heart attack in the past. He has one stent and a bad valve.
“I was happy to hear nothing was going to go inside my heart or down my arteries because I have a problem with my arteries,” he says. “The procedure went fine. Dr. Gavini really knows his stuff!”
The S-ICD procedure was approved by the Food and Drug Administration two years ago and by Medicare this year.
Dr. Gavini traveled to Minnesota to receive training from Boston Scientific in November 2014.
Those who are interested in the S-ICD system are advised to talk with their doctor about the risks and benefits associated with the procedure.
Healthy Lifestyles
- Local taxpayers to vote in November on a one percent sales tax
- Lemoore Council turns to Lemoore native Marissa Trejo as new city manager
- Lemoore City Council opts to seek solid waste proposals
- City leaders consider privatization of Lemoore's longtime refuse service
- Kings County Public Health says as of March 1, main office will not provide COVID-19 testing
- Health officials encourage community to prepare for holiday flu, COVID-19 activity
_0.jpg)

.jpg)